CBSE Class 9 – Chemistry
Page No:17
Solution 1
The condition for something to be called matter is that it should occupy space and have mass.
Solution 2
Diffusion and Brownian motion.
Solution 3
Diffusion.
Solution 4
The characteristic of matter illustrated by this observation is that the particles of matter have spaces between them.
Solution 5
This displays that each crystal of Potassium Permanganate must be made up of millions of small particles i.e. particles of matter are very very small.
Solution 6
This shows that the particles of matter are constantly moving in all direction.
Solution 7
This displays that the particles of matter attract one another. In case of chalk, the force of attraction between the particles is weak whereas the force of attraction between the particles of iron is very very strong.
Lakhmir Singh Chemistry Class 9 Solutions Page No:18
Solution 8
Atoms or Molecules.
Solution 10
(a) Solid.
(b) Liquid and Gas.
Solution 11
(a) Gases.
(b) Solids.
(c) Solids.
Solution 12
Liquid.
Solution 13
Gas.
Solution 14
Solid.
Solution 15
Gas.
Solution 16
LPG (Liquefied Petroleum Gas) and Oxygen Gas respectively.
Solution 17
(a) LPG – Liquefied Petroleum Gas.
(b) CNG – Compressed Natural Gas.
Solution 18
Gas diffuses faster.
Solution 19
Copper Sulphate into water.
Solution 20
False.
Solution 21
Diffusion.
Solution 22
Diffusion.
Solution 23
Diffusion.
Solution 24
(a) Diffusion; Brownian motion.
(b) Diffusion.
(c) States.
(d) Much more.
(e) Liquid; Gaseous.
Lakhmir Singh Chemistry Class 9 Solutions Page No: 19
Solution 25
(a) Diffusion:
(i) Matter is made up of tiny particles
(ii) The particles of matter are constantly moving.
(b) Brownian motion:
(i) The particles of matter are very, very small.
(ii) The particles of matter are constantly moving.
Solution 26
Robert Brown suspended extremely small pollen grains in water and observed it through a microscope. It was found that pollen grains were moving very rapidly throughout the water in a very irregular way. He also observed that warmer the water, faster the pollen grains move on the surface of water. This phenomenon is known as the ‘Brownian Motion’.
Solution 27
It shows that each potassium permanganate crystal is made up of millions of small particles and particles of water have spaces between them.
Solution 28
Both bromine gas and air is made up of tiny moving particles. When a gas jar containing air is inverted over gas jar containing bromine vapour, both bromine and air molecules move and collide with one another and bounce about in all directions due to which we see a uniform red brown colour in both the jars.
Solution 29
When salt is added to water and stirred, the tiny salt particles break off from each solid salt granule and fill up the spaces available between the particles of water and mix with them.
Solution 30
Air is a gas whose particles are very far apart and there are very weak forces of attraction between them. Extremely weak forces between particles of air can be overcome easily due to which we can move our hand in air. On the other hand, the particles of a solid plank of wood are very closely packed and there are very strong forces of attraction between the particles of wood. Hence, it needs a huge outside force to overcome the strong inter particle attractions which only a karate expert can apply.
Solution 31
If two metal blocks are bound together tightly and kept undistributed for a few years, then the particles of one metal are found to have diffused into the other metal.
Solution 32
The diffusion between solids is a very, very slow process because the particles in solids do not move from their fixed positions.
Solution 33
Solids diffuse the slowest as the particles in solids do not move from their fixed positions.
Gases diffuse the fastest as the particles in gases move very quickly in all directions.
Solution 34
The particles of gases produced by the burning of incense sticks move rapidly in all directions. They collide with the particles of air present in the room, mix with air and reach every part of the room quickly.
Solution 35
Three states of matter are:
(i) The solid state – Ice.
(ii) The liquid state – Water.
(iii) The gas state – Air.
Solution 36
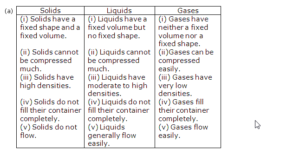
(a) Characteristics of a solid:
(i) Solids have a fixed shape and fixed volume.
(ii) Solids do not flow.
(b) Characteristics of a liquid:
(i) Liquids have a fixed volume but no fixed shape, they take the shape of the vessel in which they are placed.
(ii) They generally flow easily.
(c) Characteristics of a gas:
(i) Gases can be compressed easily.
(ii) Gases fill their container completely.
Solution 37
A gas does not have a fixed shape or fixed volume because the particles of gases do not have fixed positions or fixed spaces between them.
Solution 38
(i) Solids – They have a fixed shape and a fixed volume.
(ii) Liquids – They have a fixed volume but no fixed shape.
(iii) Gases – They neither have a fixed shape nor a fixed volume.
Solution 39
Oxygen<Water<Sugar.
Solution 40
(a) Water is a liquid at room temperature because:
(i) Water has a fixed volume (which does not change on changing its container).
(ii) Water has no fixed shape (it takes the shape of the container in which it is kept).
(b) An iron almirah is a solid because:
(i) It has a fixed shape (which cannot be changed by pressing it with hands).
(ii) It has a fixed volume (which depends on the dimensions according to which it is made).
Solution 41
(a) Diffusion.
(b) The smell of food being cooked reaches the other room by the diffusion of gases released into the air during the cooking of food.
Solution 42
(a) Diffusion in gases shows that their particles move very quickly in all directions and the rate of diffusion of a gas depends on its density. Light gases diffuse faster than heavy gases.
(b) Gases like carbon dioxide and oxygen present in the atmosphere diffuse into water (of ponds, lakes etc) and dissolves in it.
Solution 43
The smell of hot sizzling food reaches us quickly as compared to cold food because the rate of diffusion of hot gases (released by hot sizzling food) into air is faster than that of cold gases released by cold food.
Solution 44
The smell of food being cooked reaches us even from a considerable distance is because of the process of diffusion.
Solution 45
The smell of perfume spreads due to the diffusion of perfume vapours into the air.
Solution 46
The spreading of blue colour of copper sulphate into water, on its own, is due to the diffusion of copper sulphate particles into water.
Solution 47
The force of attraction between the particles of honey is much more than the force of attraction between the particles of water.
Solution 48
(a) Air is used to inflate tyres because when we blow air into a tyre the air particles push the tyre walls from inside and exerts pressure on them.
(b) Steel is used to make railway lines because steel is a rigid object having a definite shape and definite volume.
Solution 49
Diffusion occurs more quickly in gases than in a liquid because the particles in gases move very quickly in all directions whereas the particles in liquids move slowly as compared to the gas particles.
Lakhmir Singh Chemistry Class 9 Solutions Page No: 20
Solution 50
(a) The spreading out and mixing of a substance due to the motion of its particles is called diffusion. For example: Smell of food being cooked in the kitchen reaches us even from a considerable distance.
(b) Gases diffuse very fast because the particles in gases move very quickly in all directions.
(c) Carbon dioxide and Oxygen gas dissolve in water by diffusion. This process is important as these gases are essential for the survival of aquatic plants and animals. The aquatic plants use the dissolved carbon dioxide for preparing food by photosynthesis and aquatic animals use the dissolved oxygen in water for breathing.
Solution 51
(b) (i) Wood is a rigid object which has a tendency to maintain its shape when subjected to outside force.
(ii) It has a definite shape and definite volume.
Solution 52
(a) Because of high energy and negligible forces of attraction, the particles of a gas move with high speed in all directions. Thus, the pressure exerted by a gas is due to the constant collisions of the fast moving gas particles against the walls of the container.
(b) The particles of a gas have high kinetic energy and negligible forces of attraction amongst them. Due to this, the particles of a gas are constantly moving with high speeds in all the directions and the gas completely fills the vessel in which it is kept.
(c) Gases can be compressed easily because its particles are far apart and there are large spaces between them (which can be reduced by compression).
Solution 53
(a) Anything which occupies space and has mass is called matter.
Examples: Air, water, sugar, iron.
(b) The characteristics of matter are:
(i) The particles of matter are very, very small.
(ii) The particles of matter have spaces between them.
(iii) The particles of matter are constantly moving.
(iv) The particles of matter attract each other.
Solution 54
(a) The zig-zag movement of small particles suspended in a liquid (or gas) is called Brownian motion. Brownian motion increases on increasing the temperature.

(b) These dust particles move in a haphazard way because they are constantly hit by the fast moving particles of air.
Lakhmir Singh Chemistry Class 9 Solutions Page No: 21
Solution 66
(a) The red brown gas will diffuse from jar A into colorless gas in jar B due to which its red brown colour will also spread into jar B.
(b) Diffusion (in gases).
(c) Bromine vapour.
(d) Air.
(e) Potassium permanganate and water.
Solution 67
Bromine diffuses slowly into air because the motion of bromine molecules is obstructed due to the collisions with the moving molecules of air. Bromine diffuses very rapidly into vacuum because there is ‘nothing’ in the vacuum to oppose the motion of bromine molecules.
Solution 68
Chlorine will diffuse faster than bromine vapour. This is because light gases diffuse faster than heavy gases.
Solution 69
The molecules in a liquid (the brake oil) can move freely without being compressed much and hence transmit the pressure applied on brake pedal to the brake drum (on moving wheel) efficiently.
Solution 70
The steam is gaseous form of water. The molecules of water in steam move very rapidly in all directions and fill the whole kitchen space with steam. Gases (including steam) fill their container completely.
Solution 71
In both diffusion as well as osmosis, there is movement of particles from a region of higher concentration to a region of lower concentration. Diffusion can take place without there being a membrane or through a permeable membrane. But, Osmosis can take place through a semi-permeable membrane.
(a) Osmosis
(b) Diffusion
(c) Osmosis
(d) Osmosis
(e) Osmosis
(f) Diffusion
(g) Diffusion
Lakhmir Singh Chemistry Class 9 Solutions Page No: 22
Solution 72
No, the student’s conclusion is wrong. The air from the upper jar also diffuses down into the lower gas jar containing bromine vapour. But since the air is colourless it cannot be noticed by the student.
Solution 73
The fast moving molecules of air trapped in the inflated balloon exert continuous pressure on the thin, stretched rubber sheet of balloon and keep on diffusing out gradually through it.
Solution 74
(a) Pollen Grains.
(b) Water.
(c) Brownian motion.
(d) The fast moving water molecules are constantly hitting particles X causing them to move in a zig-zag path.
(e) Robert Brown.
(f) The liquid Y is made up of extremely small particles which are constantly moving.
Solution 75
(a) Dust particles.
(b) Air.
(c) Brownian motion.
(d) The fast moving air molecules are constantly hitting the tiny dust particles causing them to move rapidly in a very haphazard manner.
(e) The gaseous matter ‘air’ is made up of very tiny particles which are constantly moving.
Lakhmir Singh Chemistry Class 9 Solutions Page No: 36
Solution 1
373 K.
Solution 2
270 – 273 = -3oC.
Solution 3
573 – 273 = 300oC.
Solution 4
373 + 273 = 646 K.
Solution 5
273 + 78 = 351 K
Solution 6
-273oC
Solution 7
Latent heat.
Solution 8
(a) Degree Celsius – oC
(b) Kelvin – K.
Solution 9
Temp. on Kelvin scale = Temp. on Celsius scale + 273
Solution 10
273.
Solution 11
It means that 3.34 x 105 J of heat has to be supplied to change 1 Kg of ice (at its melting point, 0oC) into water at the same temperature of 0oC.
Solution 12
It means that 22.5 x 105 J of heat is required to change 1 Kg of water (at its boiling point, 100oC) into steam at the same temperature of 100oC.
Solution 13
(a) Boiling point.
(b) Melting point.
Solution 14
Water.
Solution 15
(a) Sublimation.
(b) Sublimation.
Solution 16
Sublimation.
Solution 17
Sublimation.
Solution 18
Dry ice.
Solution 19
Since solid carbon dioxide directly changes into carbon dioxide gas (or sublimes), and does not melt to produce a liquid (like ordinary ice), it is called dry ice.
Solution 20
Lowering temperature (or cooling)
Solution 21
False.
Solution 22
Carbon dioxide (solid).
Solution 23
(a) Pressure; temperature.
(b) Released.
(c) 273.
(d) Plasma; Bose-Einstein Condensate (BEC).
(e) Plasma
Solution 24
The heat energy that has to applied to change the state of a substance is called ‘latent heat’. They are of two types:
(i) Latent heat of fusion and (ii) Latent heat of vaporization.
Solution 25
When a solid is heated, the heat energy makes its particles vibrate more vigorously. At the melting point, the particles of solid have sufficient energy to overcome the strong forces of attraction holding them in fixed positions and break to form small groups of particles. This heat energy is kinetic energy.
Solution 26
When a change of state of a substance has to take place the heat given would not raise the temperature.
Lakhmir Singh Chemistry Class 9 Solutions Page No: 37
Solution 27
The heat energy supplied to ice during the change of state (at its melting point) is all used up in overcoming (or breaking) the force of attraction between its particles without increasing its kinetic energy. Since the heat (or latent heat) supplied during the change of state does not increase the kinetic energy of the ice cubes, therefore no rise in temperature takes place. The temperature remains constant.
Solution 28
The heat energy supplied to water during the change of state (at its boiling point) is all used up in overcoming (or breaking) the force of attraction between its particles without increasing its kinetic energy. Since the heat (or latent heat) supplied during the change of state does not increase the kinetic energy of the water, therefore no rise in temperature takes place. The temperature remains constant.
Solution 29
This is due to the fact that for melting, each kilogram of ice takes its latent heat of 3.34 x 105 joules from the substance and hence cools the substance more effectively. On the other hand, water at 0o cannot take any such latent heat from the substance.
Solution 30
We would place ice in the water to cool it more quickly because the ice takes its latent heat from the water and hence cools it more effectively. On the other hand, if we keep the water on ice then the latent heat would be taken from the surrounding air hence releasing its coolness to the surrounding and not the water.
Solution 31
Steam causes more severe burns than boiling water because the steam contains more heat, in the form of latent heat, than boiling water. Hence, when steam falls on our skin and condenses to produce water, it gives out 22.5 x 105 Joules per kilogram more heat than boiling water.
Solution 32
The latent heat of fusion of ice is 3.34 x 105 J/Kg. It means that 3.34x 105 joules of heat is required to change 1 Kg of ice at its melting point of 0oC into water at the same temperature (of 0oC). This means that 1 Kg of ice at 0oC has 3.34 x 105 joules of less heat than 1 kg of water at the same temperature of 0oC.
Solution 33
1 Kg of steam at 100oC has more heat than water at the same temperature because when water changes into steam, it absorbs latent heat, but when steam condenses to form water, an equal amount of latent heat is given out.
Solution 34
It is because of the fact that steam at 100oC contains more heat, in the form of latent heat, than boiling water at 100oC. Hence, steam would give out 22.5 x 105 joules per kilogram more heat than boiling water.
Solution 35
Steam causes more severe burns than boiling water because the steam contains more heat, in the form of latent heat, than boiling water. Hence, when steam falls on our skin and condenses to produce water it gives out 22.5 x 105 joules per kilogram more heat than boiling water.
Solution 36
The temperature of a substance remains constant during the change of state because the heat gets used up in changing the state by overcoming the forces of attraction between the particles.
Solution 37
(a) Either solid (as ice) or liquid as 0oC is the melting point of ice as well as the freezing point of water.
(b) Liquid.
(c) Either a liquid or a gas (steam) as 100oC is the boiling point of water as well as the condensation temperature of steam.
(d) Gas.
Solution 38
The temperature of a substance remains constant during the change of state though heat is supplied continuously because the heat gets used up in changing the state by overcoming the forces of attraction between the particles.
Solution 39
The temperature, at which a solid substance melts and changes into a liquid at atmospheric pressure, is called melting point of the substance. The melting point of ice is 0oC.
Solution 40
The temperature, at which a liquid boils and changes rapidly into a gas at atmospheric pressure, is called boiling point of the liquid. The boiling point of water is 100oC.
Solution 41
(a) Melting – The process in which a solid substance changes into a liquid on heating is called melting.
(b) Boiling – The process in which a liquid substance changes into a gas rapidly on heating is called boiling.
Solution 42
(a) Condensation – The process of changing a gas (or vapour) to a liquid by cooling is called condensation.
(b) Freezing – The process of changing a liquid into a solid by cooling, is called freezing.
Solution 43
This happens because naphthalene balls undergo sublimation. The naphthalene balls keep on forming naphthalene vapours slowly which disappear into the air.
Solution 44
Gases can be liquefied by applying pressure and lowering temperature. The temperature needs to be lowered because when the gas is compressed too much, then heat is produced due to compression. Cooling lowers the temperature of the compressed gas and helps in liquefying it.
Solution 45
Ammonia gas is liquefied by applying high pressure and lowering the temperature of the gas. Lowering the temperature is done by continuously pouring water over the coils carrying the compressed gas.
Solution 46
There is a lot of space between the particles of a gas. If enough pressure is applied to the gas, it gets highly compressed. The particles of gas get so close together that they start attracting each other sufficiently to form a liquid. And we say that the gas has liquefied.
Solution 47
On a hot day, when our body temperature tends to rise too much, our sweat glands give out moisture (sweat) on our skin. When this sweat evaporates, it takes the latent heat of vaporization from our body hence making our body cool.
Solution 48
All water on earth does not get evaporated on hot summer days because of the high value of latent heat of vaporization of water.
Solution 49
Liquids like alcohol, petrol and perfume are volatile (which can change into vapours easily). When we apply alcohol to the back of our hand, we find that it dries up quickly and while it is drying, the hands feel cold. This happens due to the fact that to change from liquid to the vapour state, alcohol requires latent heat of vaporization. The alcohol takes this latent heat of vaporization from the hand due to which the hand loses heat and we feel cold.
Solution 50
The cooling in a desert room cooler is caused by the evaporation of water. The higher temperature on a hot day increases the rate of evaporation of water, and the dryness of air also increases the rate of evaporation of water. And due to this increased rate of evaporation of water, a desert room cooler works better on a hot and dry day.
Solution 51
The earthen pot (or matka) has a large number of extremely small pores on its walls. Some of the water kept in the earthen pot continuously keeps seeping through these pores to the outside of the pot. This water evaporates continuously by taking the latent heat of vaporization from the earthen pot and the remaining water. In this way, the earthen pot and remaining water loses heat and gets cooled.
Solution 52
We should wear cotton clothes in hot summer days because we perspire more through the pores of the skin during such days. Since, sweat is mainly water and cotton clothes are good absorber of water, they absorb the sweat quickly and expose it to the atmosphere for evaporation. The evaporation of sweat from the cotton clothes takes the latent heat of vaporization from our skin hence the skin loses heat and makes us feel cool and comfortable.
Solution 53
If the hot tea or milk is taken in a cup, then due to the narrow shape of the cup, the surface area of hot tea in the cup is comparatively small. Due to this, the evaporation of hot tea is slow; cooling caused by evaporation is less and hence the hot tea remains appreciably hot for a much longer time. On the other hand, the saucer has a large surface area due to which the tea taken in the saucer evaporates much faster, thus cooling it quickly and making it convenient to sip or drink.
Solution 54
Acetone (or perfume) is volatile in nature. When we apply it to our palm, we feel cold. This happens due to the fact that to change from liquid to the vapour state, acetone requires latent heat of vaporization. Acetone takes this latent heat of vaporization from the hand due to which the palm loses heat and feels cold.
Solution 55
The presence of water vapour in air can be demonstrated by the following experiment: We take a steel tumbler and put some well crushed ice in it. Allow the steel tumbler to stand undisturbed for about 5 minutes with the ice in it. We would observe that a large number of tiny drops of water appear on the outer surface of the steel tumbler. This happens because the air around the steel tumbler contains water vapour in it. When these water vapour come in contact with the cold, outside surface of steel tumbler, they condense to form tiny drops of liquid.
Solution 56
(a) The latent heat of fusion of a solid is the quantity of heat in joules required to convert 1 Kg of the solid (at its melting point) to liquid, without any change in temperature. The latent heat of fusion of ice is 3.34 x 105 J/Kg.
(b)
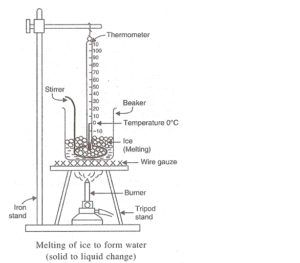
Solution 57
(a) The latent heat of vaporization of a liquid is the quantity of heat in joules required to convert 1 Kg of the liquid (at its boiling point) to vapour or gas without any change in temperature. The latent heat of vaporization of water is 22.5 x 105 J/Kg.
(b)
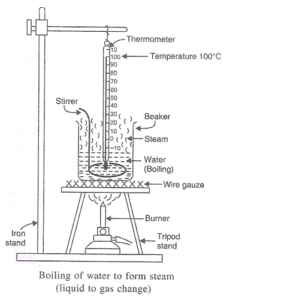
Solution 58
(a) The changing of a solid directly into vapours on heating and of vapours into solid on cooling is known as sublimation. The common substances which undergo sublimation are Camphor and Naphthalene.
(b)
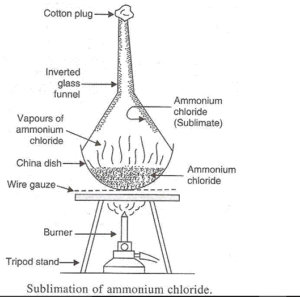
Solution 59
(a) The physical states of matter can be changed by changing pressure and changing the temperature.
(b)
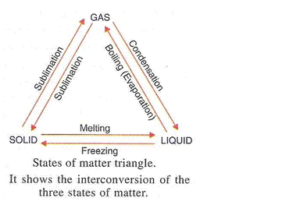
Solution 60
(a) The process of a liquid changing into vapour (or gas) even below its boiling point is called evaporation. The factors affecting rate of evaporation are:
(i) Temperature.
(ii) Surface area.
(iii) Humidity.
(iv) Wind speed.
(b) Evaporation causes cooling because when a liquid evaporates, it draws or takes the latent heat of vaporisation from ‘anything’ which it touches and hence the substances or surroundings lose heat and get cooled.
Lakhmir Singh Chemistry Class 9 Solutions Page No: 39
Solution 81
(a) (i) W – Iodine (ii) X – Sodium Chloride (iii) Y – Naphthalene (iv) Z – Ammonium chloride.
(b) W – Iodine; Y – Naphthalene; Z – Ammonium chloride.
(c) Y – Naphthalene.
(d) Tincture Iodine.
(e) W – Iodine.
Solution 82
(a) (i) Water (ii) Ice (iii) Steam.
(b) Freezing.
(c) 0oC.
(d) Boiling (or vaporisation).
(e) 100oC
Solution 83
(a) (i) Liquid (ii) Gas (iii) Solid (iv) Plasma (v) Bose-Einstein Condensate (BEC).
(b) Ammonium chloride; Sublimation.
(c) Carbon dioxide.
(d) Water.
(e) D (plasma).
Solution 84
(a) 273 K.
(b) Freezing.
(c) Latent heat of freezing.
(d) Melting.
(e) Latent heat of fusion.
Lakhmir Singh Chemistry Class 9 Solutions Page No: 40
Solution 85
(a) 373 K.
(b) Boiling (or vaporisation).
(c) Latent heat of vaporisation.
(d) Condensation.
(e) Latent heat of condensation.